Fast-Track Your Medical Device
to Market
Build regulatory-grade evidence during development — reduce prototypes, consultant dependency, and approval uncertainty.
Always answer: "Are we safe and ready to submit?"
The Platform
Three integrated products that produce a continuous evidence graph — the backbone of your regulatory submission.
Digital Twin Core
Transform device design into measurable patient safety behavior with multi-physics simulation and evidence chain storage.
Agentic Regulatory Network
Continuously audit regulatory readiness with AI agents that detect missing verification and generate submission documentation.
Confidential Expert Collaboration
Enable expert validation without exposing intellectual property through structured micro-reviews and role-based visibility.
See It In Action
Watch how MedTech Fast-Track transforms medical device development from concept to regulatory submission.
Fast-Tracking Medical Tech
A concise introduction to the MedTech Fast-Track platform vision and how it transforms medical device development.
AI-Enhanced MedTech Approval
A deeper dive into platform capabilities including Digital Twin simulation, regulatory agents, and expert collaboration.
Platform Capabilities
Explore the technical depth of each integrated component.
Digital Twin Core
Transform device design into measurable patient safety behavior.
- Import CAD, schematics, firmware logic, and materials
- Multi-physics simulation: thermal, mechanical, electrical, chemical, software
- Detect safe operating envelope early in development
- Store complete evidence chain: assumption → model → prediction → confidence → proof needed
Agentic Regulatory Network
Continuously audit regulatory readiness with four specialized AI agents.
- Safety Agent: hazard coverage analysis
- Regulatory Agent: submission completeness tracking
- Clinical Agent: outcome relevance validation
- Manufacturing Agent: reproducibility verification
- Detect missing verification and recommend fastest proof method
- Live submission readiness score
- Auto-generated documentation: risk file, traceability matrix, verification plan, submission summary

Confidential Expert Collaboration
Enable expert validation without exposing intellectual property.
- Share behavior outputs, not designs — protect your IP
- Structured micro-reviews with targeted expert feedback
- Role-based visibility and redacted models
- Consensus evidence artifacts from multiple expert reviews
- Immutable audit trail for regulatory compliance
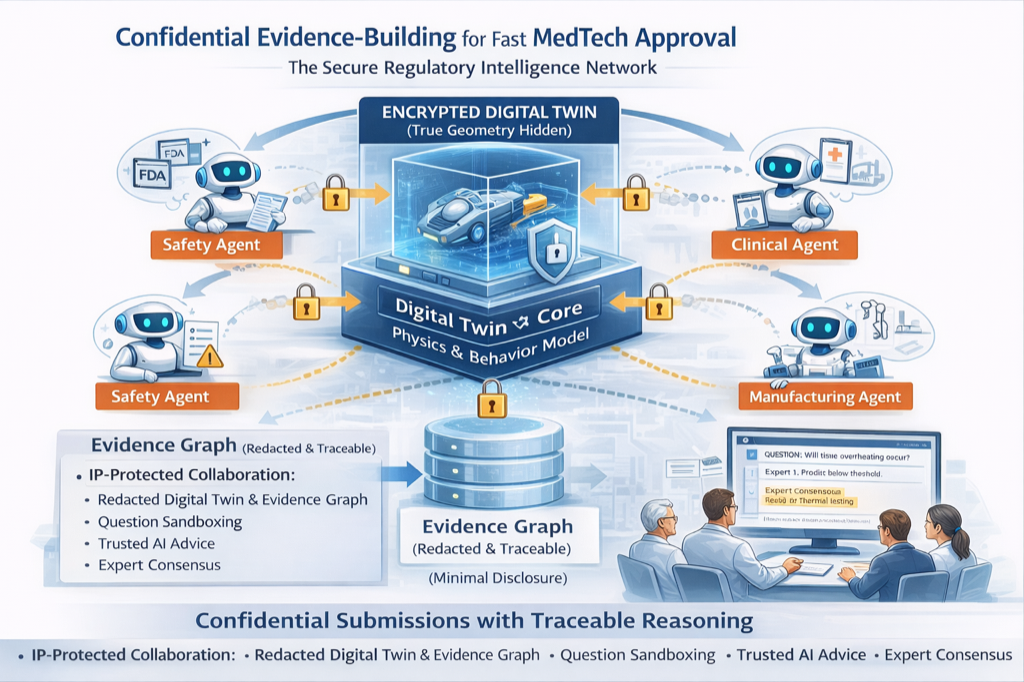
Continuous Evidence Graph
All components write to a continuous evidence chain that becomes the regulatory submission backbone.
How It Works
Four phases from device design to regulatory submission — guided by continuous evidence.
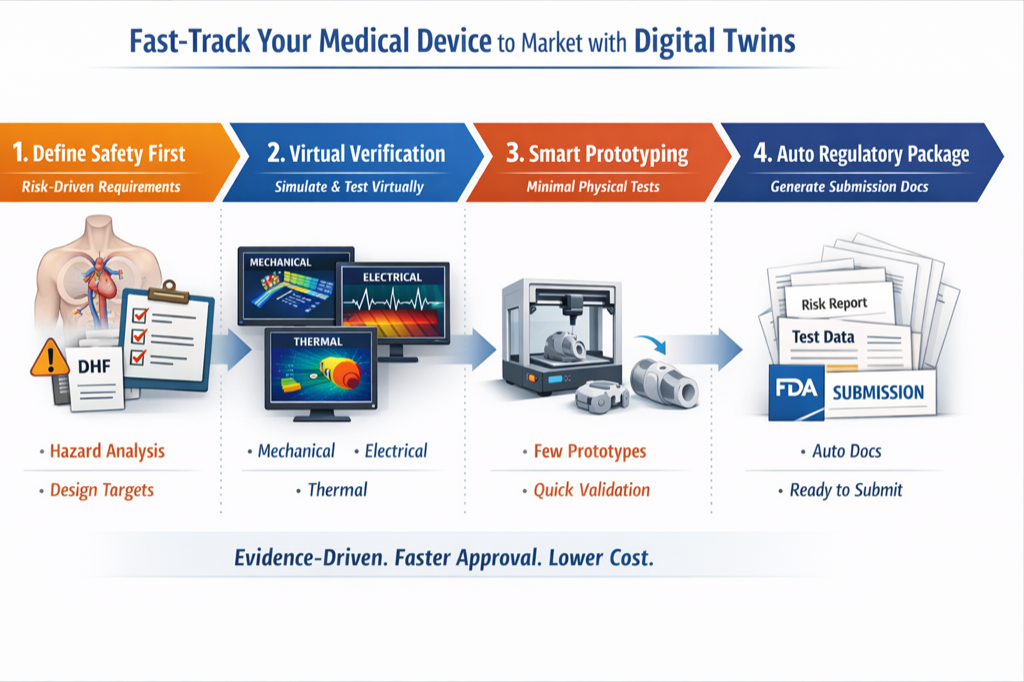
Define Safety First
Risk-Driven Requirements
- Hazard analysis & risk assessment
- Define design targets & safety criteria
Virtual Verification
Simulate & Test Virtually
- Mechanical, electrical, thermal simulation
- Detect safe operating envelope early
Smart Prototyping
Minimal Physical Tests
- Build only 1-3 targeted prototypes
- Quick validation of simulation predictions
Auto Regulatory Package
Generate Submission Docs
- Risk report & test data compilation
- FDA submission readiness & auto documentation
Evidence-Driven. Faster Approval. Lower Cost.
Why MedTech Fast-Track?
See how the platform approach compares to traditional medical device development.
| Dimension | Traditional | Platform |
|---|---|---|
| Prototypes | 6-12 prototypes | 1-3 prototypes |
| Feedback | Late-stage feedback | Continuous feedback |
| Documentation | Manual documentation | Auto evidence generation |
| Workflow | Consultant-heavy | Guided workflow |
| Approval | Regulatory uncertainty | Predictable approval path |
Prototypes
Feedback
Documentation
Workflow
Approval
MVP: What's Available Now
Start building regulatory-grade evidence today with our initial platform capabilities.
Thermal + Electrical Digital Twin
Simulate burn risk, shock hazards, and leakage current from your device design.
Regulatory Gap Agent
AI-powered detection of missing verification and submission gaps.
Expert Micro-Review Portal
Confidential expert validation with IP-protected behavior sharing.
Auto Traceability
Automatically generated traceability matrix and evidence chain documentation.
Get Early Access
Interested in transforming your medical device development? Tell us about your project.